Guz,
Would you be willing to explain "contact angle"? I've begun to see the term used a bit and would like to know more.
Thanks!
Check out this video for the concept. About 1:10 into
Pulled from IGL.
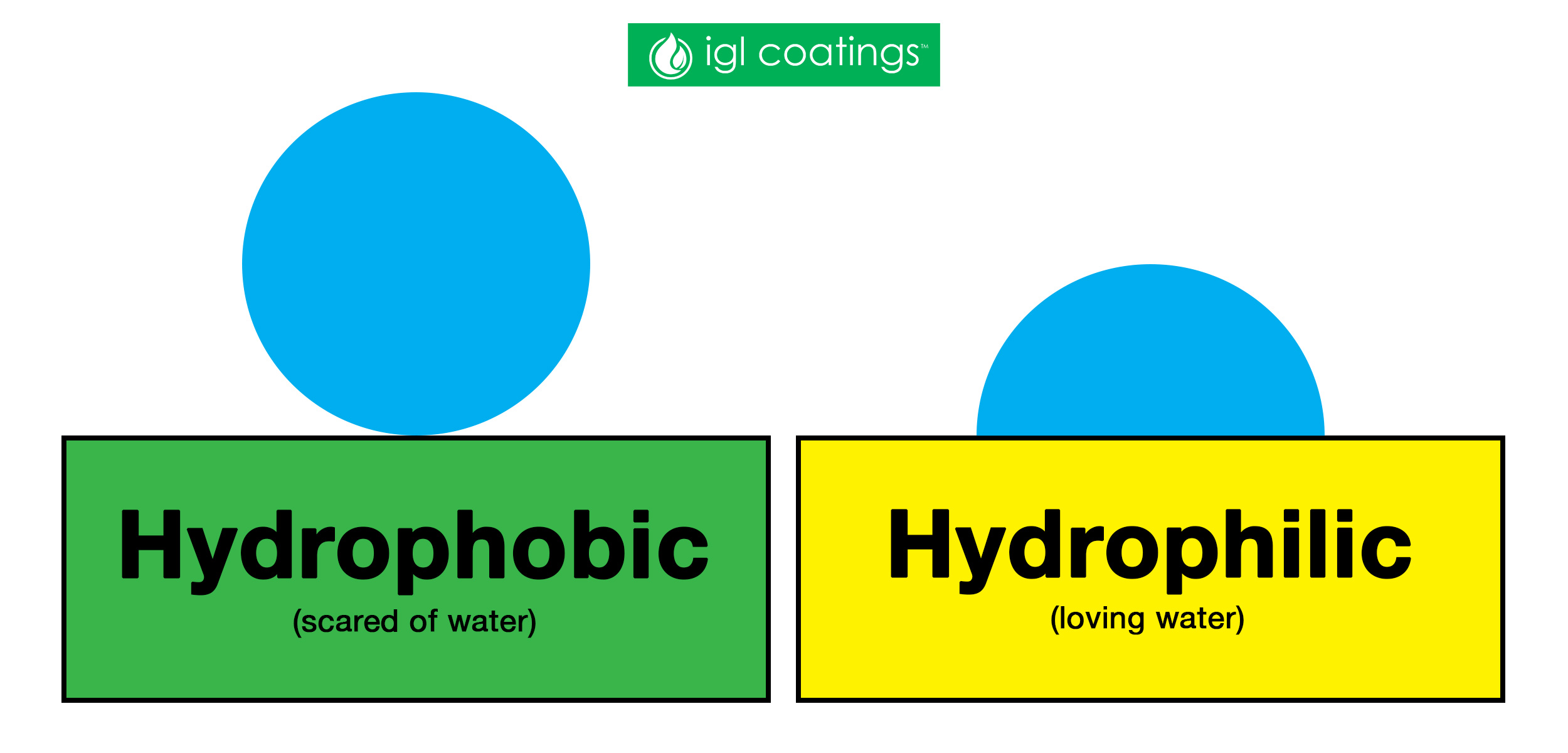
The common behaviour of liquids is that it has a tendency to stick to things, including itself. Therefore, a surface is classified as either hydrophobic (scared of water) or hydrophilic (loving water) by the way it interacts with liquid, specifically water droplets.
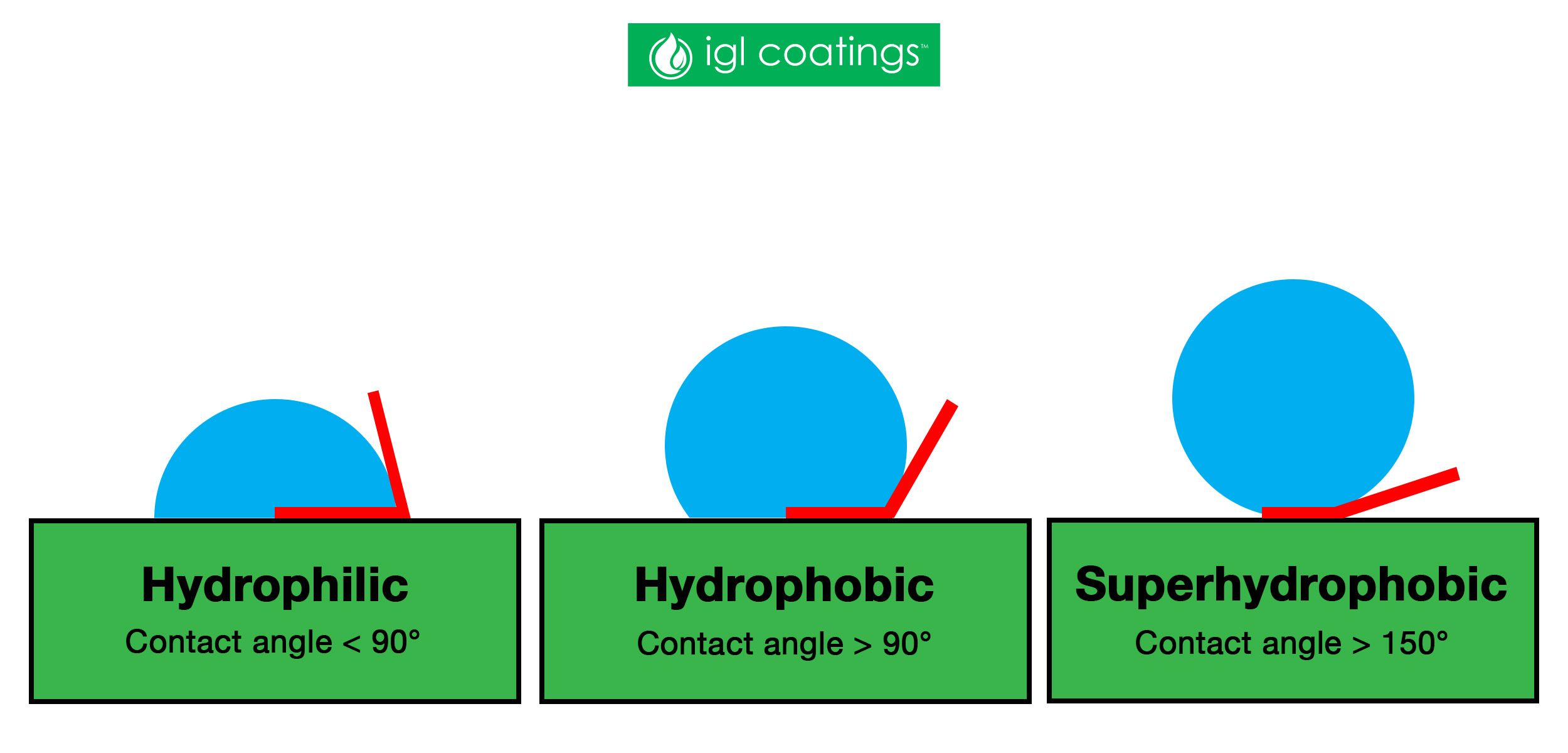
A droplet that has absolutely no interaction with a surface will bead up into a perfect sphere and will have a contact angle of 180°whilst a water droplet that completely wets the surface has a contact angle of 0°.
Contact Angle Significance
If the water droplet sticks more to the surface than itself, that surface is hydrophilic and the water droplet will have a contact angle of less than 90°.
If the water droplet has a tendency to stick to itself more than it sticks to the surface, that surface is hydrophobic and water droplets will bead up to a contact angle of more than 90°.
The term superhydrophobic (also ultrahydrophobic) occurs when the effective contact angle of water drops is 150° or higher. There are many examples of naturally occurring superhydrophobic surfaces. These include both plants and insects surfaces.
One of the most notable superhydrophobic surface in nature is that of the lotus leaf. The extreme water repellency exhibited by lotus leaves is what inspired scientists to initially pursue research into superhydrophobic phenomena.
Superhydrophobic is used to describe a surface that exhibits both advancing and receding contact angles of higher than 150°.
The angle at which a water drop rolls off a tilted flat surface is known as the roll-off angle. Generally, superhydrophobic surfaces exhibit roll-off angles less than 5°, but very high quality superhydrophobic surfaces can exhibit roll-off angles of less than 1°.

 Lol.
Lol.